Charging your phone can be an exercise in patience that many of us are just not in the mood for.
Imagine if you could fully charge your cell phone in a matter of seconds? Researchers at Drexel University’s College of Engineering are able to thanks to their recent work unveiling of a new battery electrode design in the journal Nature Energy, which utilises a highly conductive material called MXene.
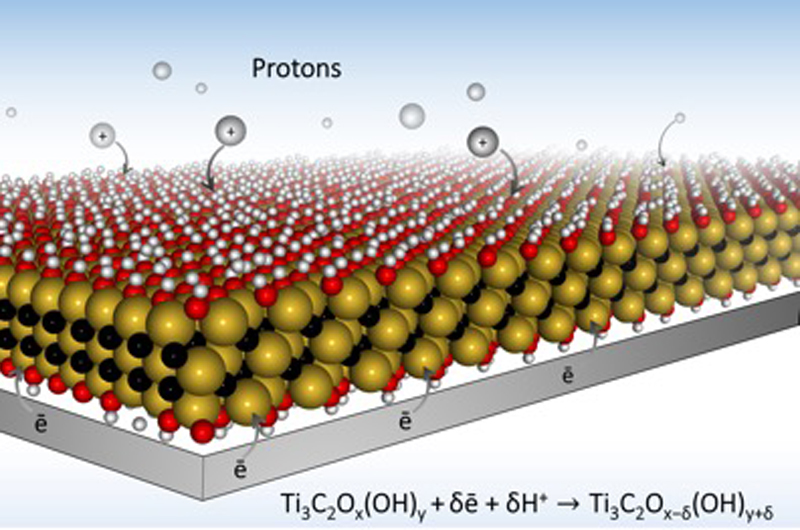
“This paper refutes the widely accepted dogma that chemical charge storage, used in batteries and pseudocapacitors, is always much slower than physical storage used in electrical double-layer capacitors, also known as supercapacitors,” Gogotsi said. “We demonstrate charging of thin MXene electrodes in tens of milliseconds. This is enabled by very high electronic conductivity of MXene. This paves the way to development of ultrafast energy storage devices than can be charged and discharged within seconds, but store much more energy than conventional supercapacitors.”
The key to charging batteries faster lies is in the electrode design. Electrodes are essential components of batteries, because it’s through them energy is stored when charging and where energy is disbursed from in order to power your devices.
MXenes are revolutionary because they create multiple paths for ions to travel through, which means that more of them can get to the charging ports at a faster rate. This is due to the composition of MXene itself. Because MXene is highly conducive, the ions are able to move along without much resistance. MXene also has more ports than traditional batteries, this means that there are more places for ions to enter and leave the battery.
“In traditional batteries and supercapacitors, ions have a tortuous path toward charge storage ports, which not only slows down everything, but it also creates a situation where very few ions actually reach their destination at fast charging rates,” said Lukatskaya, the first author on the paper, who conducted the research as part of the A.J. Drexel Nanomaterials Institute. “The ideal electrode architecture would be something like ions moving to the ports via multi-lane, high-speed ‘highways,’ instead of taking single-lane roads. Our macroporous electrode design achieves this goal, which allows for rapid charging — on the order of a few seconds or less.”
At this stage there’s no word on whether or not this technology will be widely available in the near future, but the potential for MXene is incredibly exciting.



